A clinically studied, standardised fenugreek seed extract rich in furostanolic saponins Furosap® is a highly standardised extract of fenugreek seed (Trigonella foenum-graecum L.), developed to deliver a consistent and well-characterised profile of furostanolic saponins, including the key marker compound protodioscin. Fenugreek has a long history of traditional use, but Furosap® represents a modern, evidence-driven approach — combining botanical heritage with contemporary analytical testing and human clinical research. Botanical name: Trigonella foenum-graecum L. Plant part used: Seed Extract type: Standardised fenugreek seed extract Natural plant materials can vary due to growing conditions, harvest timing, and processing. To address this variability, Furosap® is standardised to defined active fractions to ensure batch-to-batch consistency and alignment with the material evaluated in published human studies. The extract is characterised for: Furosap® is assessed using laboratory techniques routinely applied to high-quality botanical extracts. These values are determined using high-performance liquid chromatography (HPLC), ensuring the extract consistently meets its defined specification. Furosap® is characterised using analytical techniques commonly applied to high-quality botanical extracts to confirm identity, composition, and consistency. The extract is standardised to defined levels of furostanolic saponins, including the key marker compound protodioscin. These compounds are quantified using high-performance liquid chromatography (HPLC), allowing precise measurement of active constituents and ensuring consistency with the extract profile used in published human studies. Chromatographic analysis produces a characteristic chemical “fingerprint” for the extract. Across repeated analyses, the key marker compounds appear at consistent positions with stable relative intensity. This demonstrates that the extract behaves predictably during testing and maintains a repeatable composition from batch to batch. In addition to active compound verification, the ingredient is assessed against defined quality criteria, including: Together, these measures confirm that Furosap® meets its defined specification and is suitable for use in high-quality supplement formulations. Human clinical studies conducted by academic and clinical research groups in India, and published in peer-reviewed journals such as the International Journal of Medical Sciences, have evaluated Furosap® in healthy adult men. Clinical investigations carried out by medical researchers working with hospital-based laboratories examined sperm parameters in healthy volunteers. Reported outcomes included improvements in: A randomised, double-blind, placebo-controlled study published in Functional Foods in Health and Disease investigated Furosap® supplementation in resistance-trained men. Furosap® has also been evaluated within registered clinical trial frameworks, including listings on ClinicalTrials.gov, reflecting transparent and structured investigation under recognised research standards. Across published human studies, Furosap® has shown a favourable safety profile when consumed at the studied intake levels. Safety assessments included: No serious adverse effects were reported in healthy adult populations during the study periods. Most human clinical studies investigating Furosap® have used a daily intake of 500 mg of the standardised extract, consumed consistently over periods of 8–12 weeks. For those seeking a fenugreek extract supported by both scientific research and robust quality verification, Furosap® represents a modern, evidence-based option. Furosap® is available as a single-ingredient formulation here.Furosap® (Fenugreek Seed Extract)
Introduction
Botanical identity and standardisation
Composition and analytical quality
Key characteristics
Active compound profile
Analytical Characterisation & Ingredient Verification

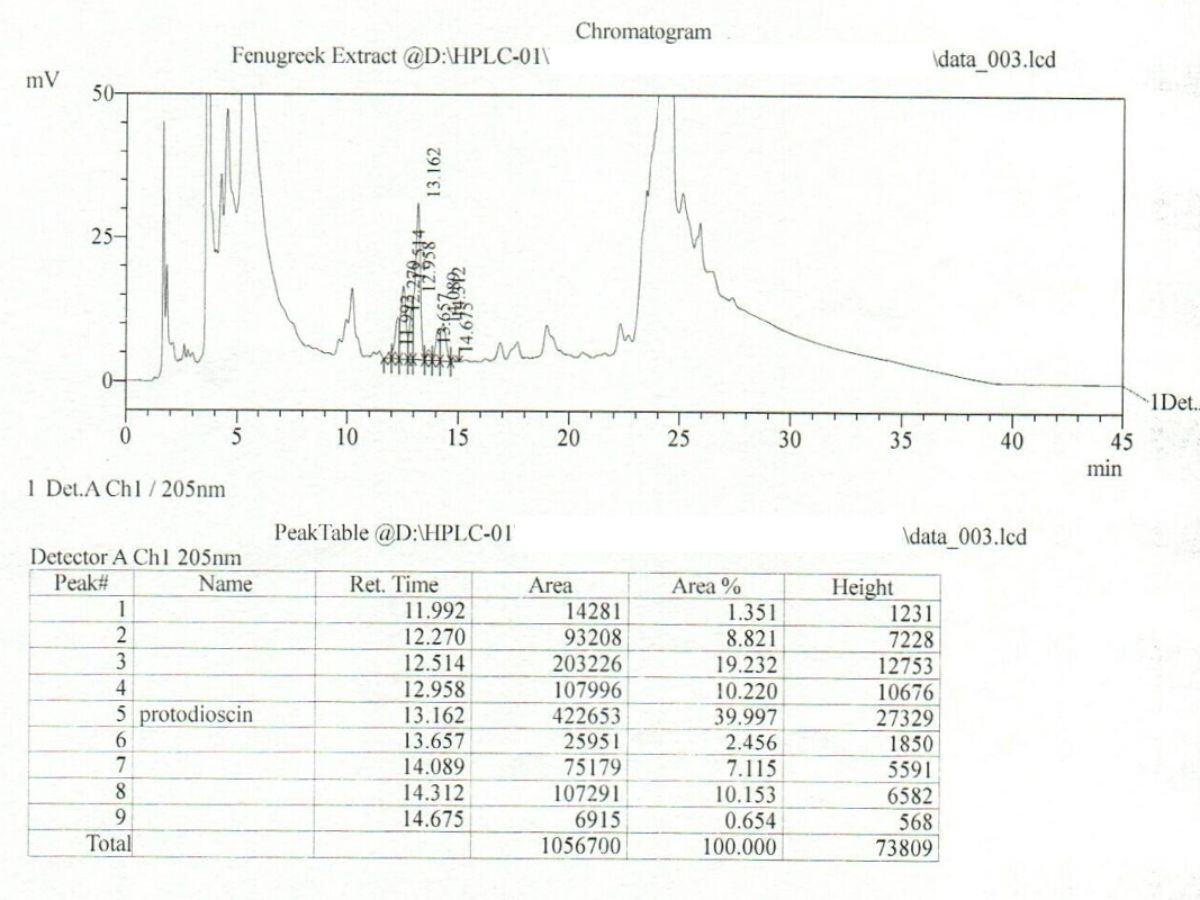
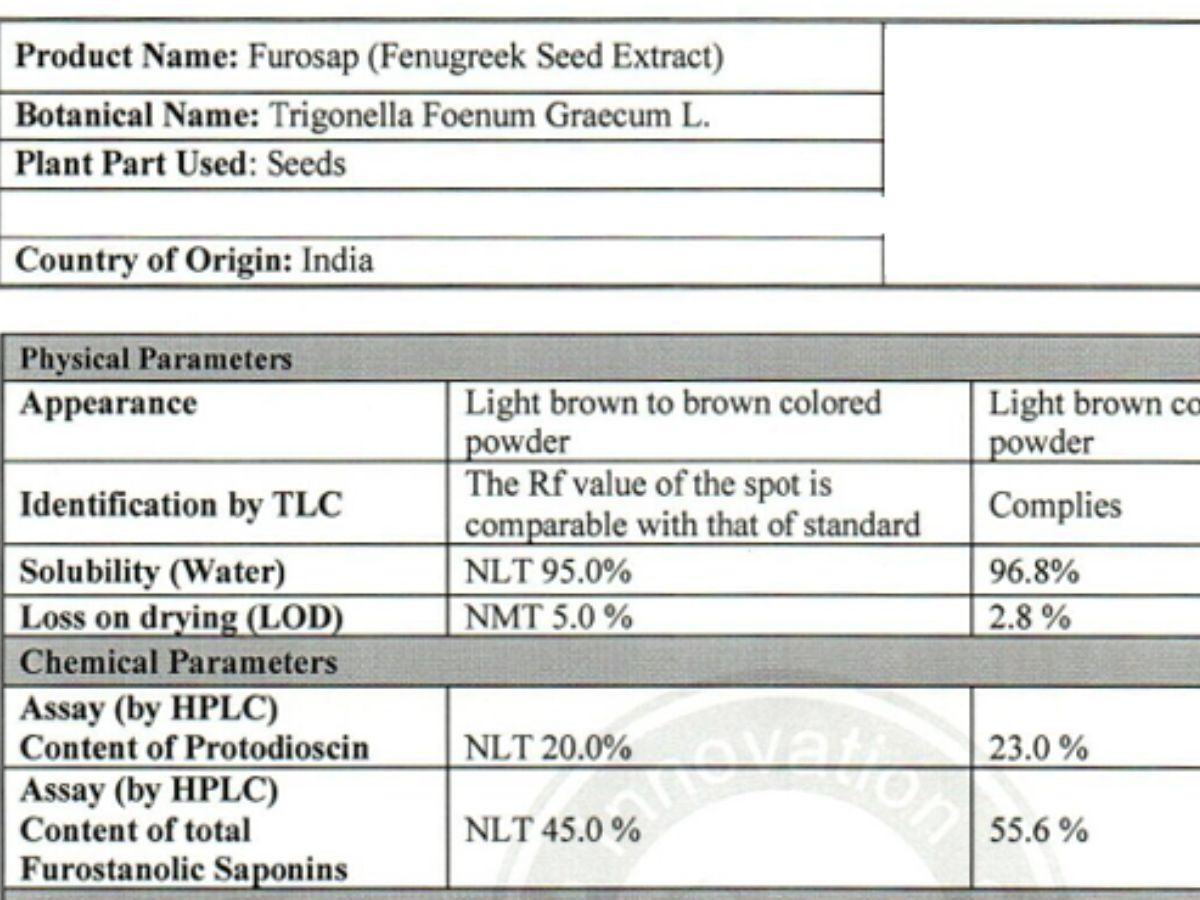
Active compound profile
Consistency and repeatability
Identity, purity, and quality controls
Human clinical research
Testosterone and male vitality
Male reproductive health
Body composition and physical performance
Registered clinical evaluation
Safety and tolerability
Potential benefits (evidence-led)
Typical intake used in studies
Summary
References
