A clinically evaluated green coffee bean extract standardised to 70% chlorogenic acids GCB70® is a standardised green coffee bean extract derived from Coffea species and enriched to contain high levels of chlorogenic acids, a group of naturally occurring polyphenols found in unroasted coffee beans. Unlike roasted coffee, where chlorogenic acids are significantly reduced, green coffee bean extracts provide a concentrated source of these bioactive compounds. Green coffee bean extracts have been studied for their potential role in supporting body weight management, metabolic balance, and glycaemic markers. GCB70® has been evaluated in human clinical research exploring changes in body weight, body composition, waist circumference, and metabolic parameters in overweight individuals. Green coffee beans are naturally rich in chlorogenic acids, a family of polyphenolic compounds that include several caffeoylquinic acid isomers. GCB70® is standardised to ensure a consistent chlorogenic acid content, allowing alignment with material evaluated in human studies. GCB70® is characterised using analytical techniques commonly applied to botanical extracts intended for food supplement use. Active constituents are quantified using high-performance liquid chromatography (HPLC), ensuring the extract consistently meets its defined specification. GCB70® is assessed against defined identity, purity, and quality criteria to confirm suitability for use in high-quality supplement formulations. Botanical identity is verified using thin-layer chromatography (TLC), with results confirming the presence of chlorogenic acids characteristic of green coffee bean extracts. Chromatographic analysis produces a characteristic chemical “fingerprint” for the extract. Across repeated analyses, key chlorogenic acid components appear at consistent positions and relative intensities, demonstrating a repeatable and stable composition from batch to batch. In addition to active compound verification, the ingredient is assessed against established quality parameters, including: Together, these measures confirm that GCB70® meets its defined specification and maintains a consistent analytical profile suitable for food supplement use. A controlled human clinical evaluation investigated the effects of GCB70® supplementation in overweight adults over a 12-week period. Participants consumed a total daily intake of 1,000 mg of the extract. Reported outcomes included: All reported changes were statistically significant, and standard blood chemistry parameters remained within normal ranges throughout the study. No serious adverse effects were reported during the intervention period, supporting a favourable tolerability profile for GCB70® when used at the studied intake level. Based on its composition and available human research, GCB70® may support: These outcomes reflect findings from controlled human research and are dependent on overall dietary and lifestyle context. Human clinical evaluation of GCB70® has used a daily intake of 1,000 mg, consumed consistently over a 12-week period. GCB70® is a well-characterised, standardised green coffee bean extract enriched to ≥70% chlorogenic acids. Analytical verification confirms a consistent chlorogenic acid profile, low caffeine content, and compliance with purity and safety criteria. Human clinical research has demonstrated statistically significant improvements in body weight, BMI, waist circumference, and selected metabolic markers, alongside a favourable safety profile. For those seeking a green coffee bean extract supported by analytical rigour and human clinical evaluation, GCB70® represents a transparent and evidence-based option. GCB70® is available as a single-ingredient formulation here.GCB70® (Green Coffee Bean Extract)
Introduction
Botanical identity and standardisation
Composition and analytical quality
Key physical characteristics
Active compound profile
Analytical Characterisation & Ingredient Verification

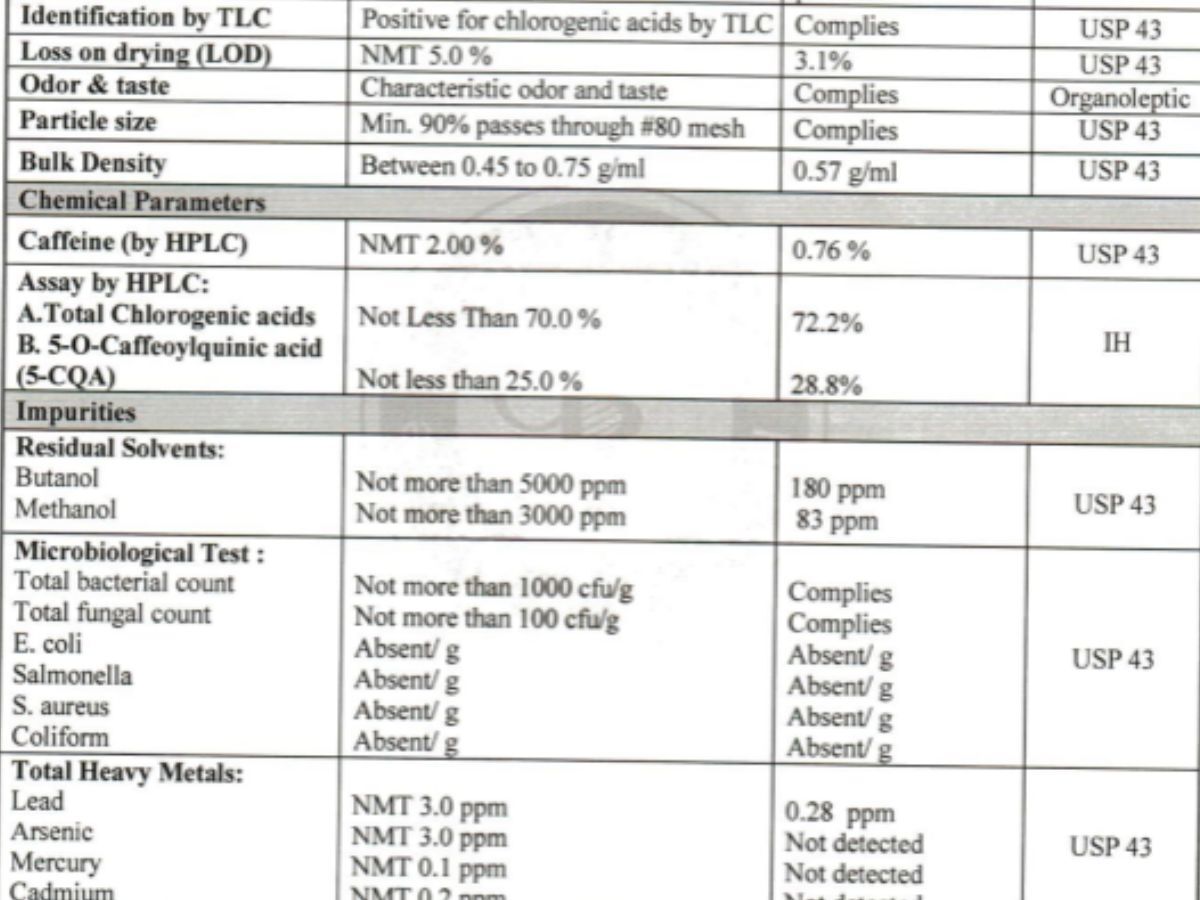
Identity confirmation
Consistency and repeatability
Purity and quality controls
Human clinical research
Body weight and metabolic outcomes
Safety observations
Potential benefits (evidence-led)
Typical intake used in studies
Summary
