A standardised Tongkat Ali root extract produced using patented ultrasonic hot water extraction Malaysian Tongkat Ali extract is derived from the root of Eurycoma longifolia, a flowering plant native to Southeast Asia and traditionally used in Malaysia for male vitality, physical performance, and general wellbeing. The root has a long history of ethnobotanical use and has become the focus of modern scientific investigation due to its unique profile of bioactive compounds. This extract is produced using a patented ultrasonic hot water extraction process, designed to efficiently release water-soluble bioactives from the root while avoiding harsh organic solvents. The ingredient has been analytically characterised and evaluated in collaboration with the Forest Research Institute Malaysia (FRIM), supporting its identity, composition, and quality profile. The use of hot water extraction, enhanced by ultrasonic processing, allows for efficient cell wall disruption and improved recovery of key bioactive constituents while maintaining a clean extraction profile suitable for food supplement applications. Malaysian Tongkat Ali extract is characterised for a defined group of bioactive compounds commonly associated with Eurycoma longifolia root. Active compound profile Eurycomanone is a quassinoid compound widely used as a chemical marker for Tongkat Ali identity and extract quality. Glycosaponins represent a broader class of compounds contributing to the extract’s overall biological profile. This Tongkat Ali extract is assessed against defined identity, purity, and quality criteria to confirm suitability for use in high-quality supplement formulations. Botanical identity is verified through chromatographic techniques that demonstrate the presence of characteristic Tongkat Ali compounds. Qualitative UPLC analysis confirms distinct peaks corresponding to eurycomanone, with retention times consistent with authenticated reference standards. Quantitative chromatographic analysis confirms a defined active profile aligned to specification, including: Standard-curve analysis of eurycomanone supports analytical reliability and consistent quantification of this key marker compound. Chromatographic “fingerprint” profiles show consistent peak patterns across analyses, indicating a repeatable extraction process and controlled composition from batch to batch. This consistency reflects both raw material selection and the stability of the ultrasonic hot water extraction method. Tongkat Ali root extracts have been investigated in human research, particularly in relation to male vitality, physical performance, stress adaptation, and hormonal balance. Studies have explored outcomes including changes in testosterone-related markers, body composition, mood, and perceived wellbeing. Eurycomanone is commonly used as a marker compound for extract potency and authenticity and is frequently referenced in analytical and research contexts relating to Eurycoma longifolia. Human studies and long-standing traditional use indicate that Eurycoma longifolia root extracts are generally well tolerated when consumed by healthy adults at commonly studied intake levels. Reported adverse effects are typically mild and infrequent. No serious safety concerns have been consistently identified in the published literature when extracts are appropriately manufactured and consumed as part of a balanced diet and healthy lifestyle. These potential benefits are supported by human research on Eurycoma longifolia extracts and are influenced by overall lifestyle, diet, and individual context. Malaysian Tongkat Ali extract is commonly formulated in daily servings that deliver a defined eurycomanone intake, consistent with levels used in human research and traditional applications. Malaysian Tongkat Ali extract is a standardised Eurycoma longifolia root extract produced using a patented ultrasonic hot water extraction process. Analytical characterisation, including collaborative work with FRIM, confirms a defined bioactive profile containing 2–3% eurycomanone and 30–40% glycosaponins, alongside appropriate purity and safety parameters. With its controlled composition, repeatable analytical profile, and alignment with a substantial body of human research on Tongkat Ali, this extract represents a high-quality, evidence-led ingredient for formulations focused on male vitality, performance, and wellbeing. Malaysian Tongkat Ali is available as a single-ingredient formulation here.Malaysian Tongkat Ali Extract (Eurycoma longifolia Root Extract)
Introduction
Botanical identity and standardisation
Composition and analytical quality
Analytical Characterisation & Ingredient Verification

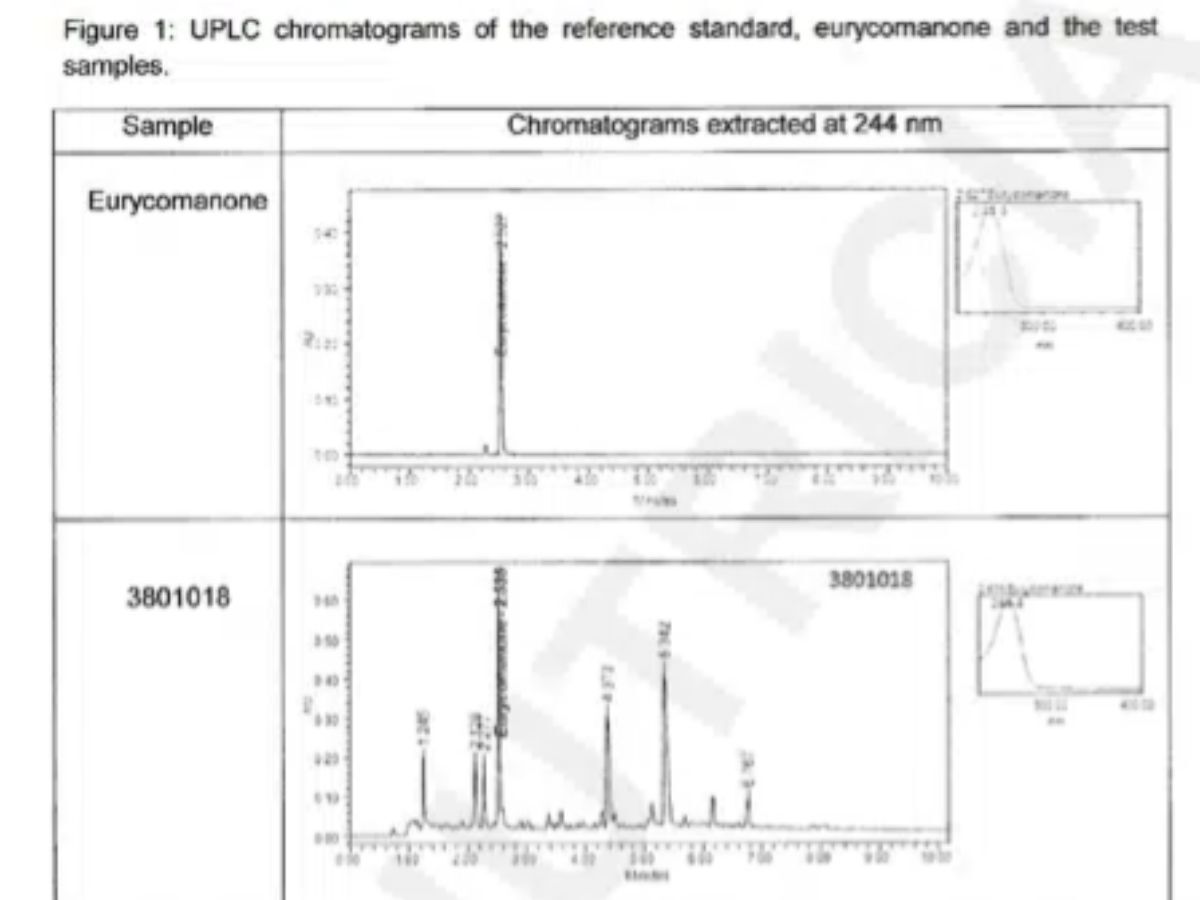

Identity confirmation
Active compound verification
Consistency and repeatability
Physical quality markers
Microbiological quality
Heavy metals
Human research context
Safety and tolerability
Potential benefits (evidence-led)
Typical use context
Summary
