A high-purity NMN powder verified by chromatographic identity testing NMN (β-nicotinamide mononucleotide) is a naturally occurring nucleotide involved in cellular metabolism. NMN is a direct precursor to NAD+ (nicotinamide adenine dinucleotide), a coenzyme required for many biological processes related to energy metabolism and cellular function. As a supplement ingredient, NMN is typically used by individuals seeking support for healthy ageing and metabolic wellness as part of a balanced diet and lifestyle. NMN is assessed using high-performance liquid chromatography (HPLC) to confirm identity and quantify purity. This provides a consistent and reproducible method for confirming the composition of the finished powder. NMN powder is verified against defined identity, purity, and safety parameters to confirm suitability for food supplement use. Chromatographic identity testing confirms the NMN peak matches the reference standard, and quantitative HPLC analysis confirms high purity. Typical results indicate: Quality testing includes screening for heavy metals, residual solvents, and pesticide residues against defined limits suitable for food supplement raw materials. Typical results include: Microbiological testing confirms low total counts and absence of relevant pathogens. Typical results include: NMN has been investigated in human research in relation to NAD+ metabolism and markers associated with healthy ageing. The research literature continues to develop, and outcomes vary depending on study design, population, and dose. NMN has been evaluated in human studies at a range of intake levels. As with any supplement ingredient, suitability depends on the individual, overall diet, and health context. Individuals who are pregnant, breastfeeding, taking medication, or managing a medical condition should seek professional advice before use. NMN powder is a high-purity β-nicotinamide mononucleotide ingredient verified by chromatographic identity testing and quantified by HPLC. Quality assessment includes screening for contaminants and microbiological parameters, with independent testing confirming >99% NMN. This analytical approach supports consistency, transparency, and suitability for food supplement use.NMN Powder (β-Nicotinamide Mononucleotide)
Introduction
Ingredient identity and specification
Analytical Characterisation & Ingredient Verification

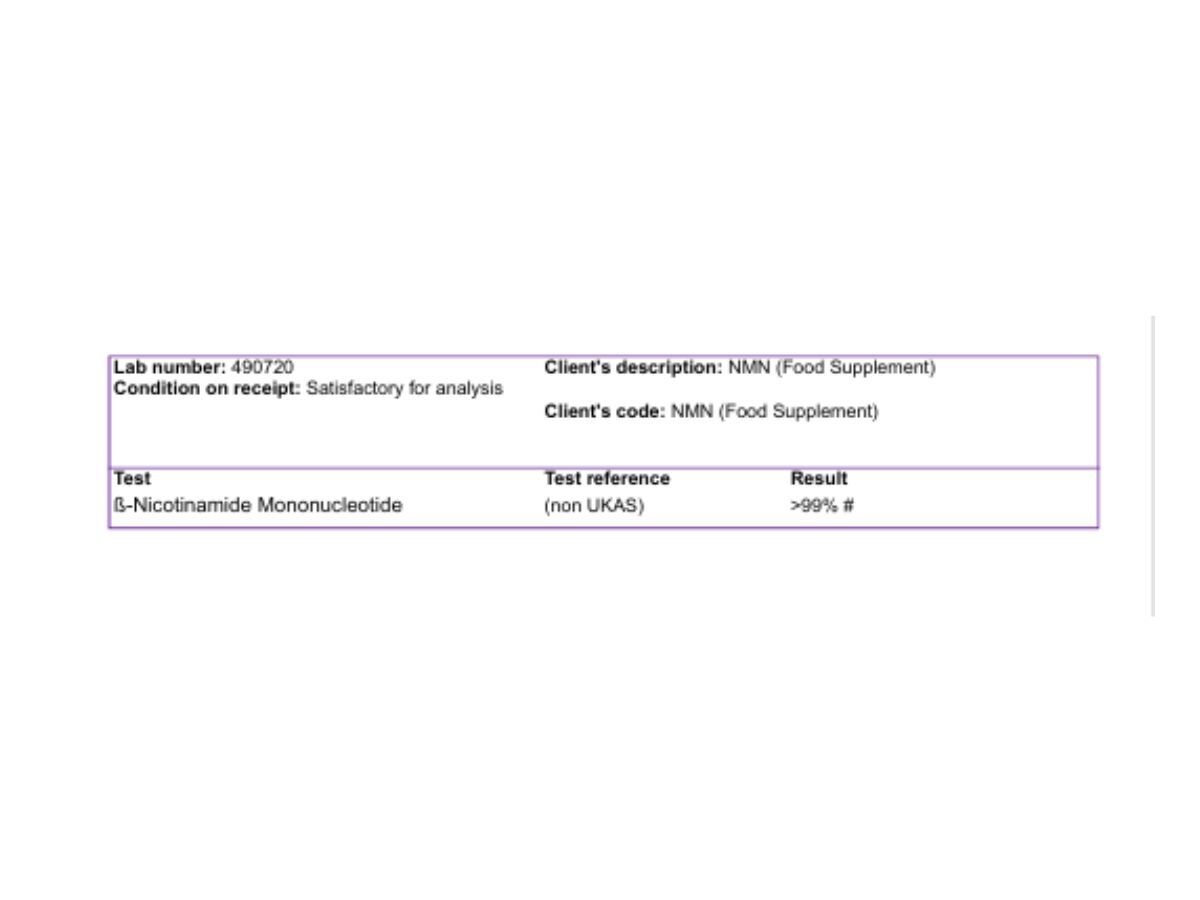
Identity and purity (HPLC)
Physical quality markers
Contaminants and residues
Microbiological quality
Human research context
Safety and tolerability
Potential benefits (evidence-led)
Summary
